Single-Port Robotic Areolar Thyroidectomy: How I Do It
Article information
Abstract
With the recent development of the da Vinci Single Port (SP) robotic surgical system, new surgical methods applying the da Vinci SP in thyroid surgery are being reported. We first reported a method known as single-port robotic areolar (SPRA) thyroidectomy in 2023, and we performed more than 100 SPRA thyroidectomies in a year. SPRA is a more minimally invasive method than the existing bilateral axillary breast approach method, as the subcutaneous flap area is reduced by more than 50%. Herein, we present a step-by-step description of the method of SPRA thyroidectomy.
Introduction
According to the National Cancer Information Center [1], thyroid cancer is the most common cancer, ranking first in incidence each year. It predominantly affects women, with a male-to-female sex ratio of 0.3:1, and is most frequently diagnosed in individuals aged 30 to 50 years [1]. Therefore, various surgical techniques have been developed to avoid leaving visible scars on the neck. Initially, remote-access thyroid surgery was performed using an endoscopic approach. Following the introduction of robotic surgical systems, most remote access thyroid operations have transitioned to robotic techniques [2]. More recently, with the introduction of the da Vinci Single Port (SP), initial reports have emerged on single-port robotic thyroid surgery employing a trans-axillary approach [3,4]. The authors have developed a novel surgical method known as single-port robotic areolar (SPRA) thyroid surgery, which forgoes the axillary approach used in the previous bilateral axillary breast approach (BABA) method [5]. In this section, we present a detailed description of total thyroidectomy with central lymph node dissection using SPRA.
Case Presentation
A 35-year-old woman was diagnosed with left papillary thyroid carcinoma (PTC), with the tumor measuring 6 mm. A preoperative computed tomography scan indicated suspected metastasis in the left central lymph node. She was scheduled for SPRA thyroid surgery. During the procedure, a frozen biopsy of the central lymph node revealed metastatic PTC in five out of eight nodes at level 6, prompting a total thyroidectomy. The final pathology confirmed PTC, located on the left, measuring 6 mm, with microscopic extrathyroidal extension and metastasis in six of the 22 central lymph nodes. Postoperatively, the patient’s vocal cord function remained normal, and she did not develop hypocalcemia. Three months post-surgery, just prior to radioactive iodine treatment, the thyroid-stimulating hormone–stimulated thyroglobulin level was below 0.2.
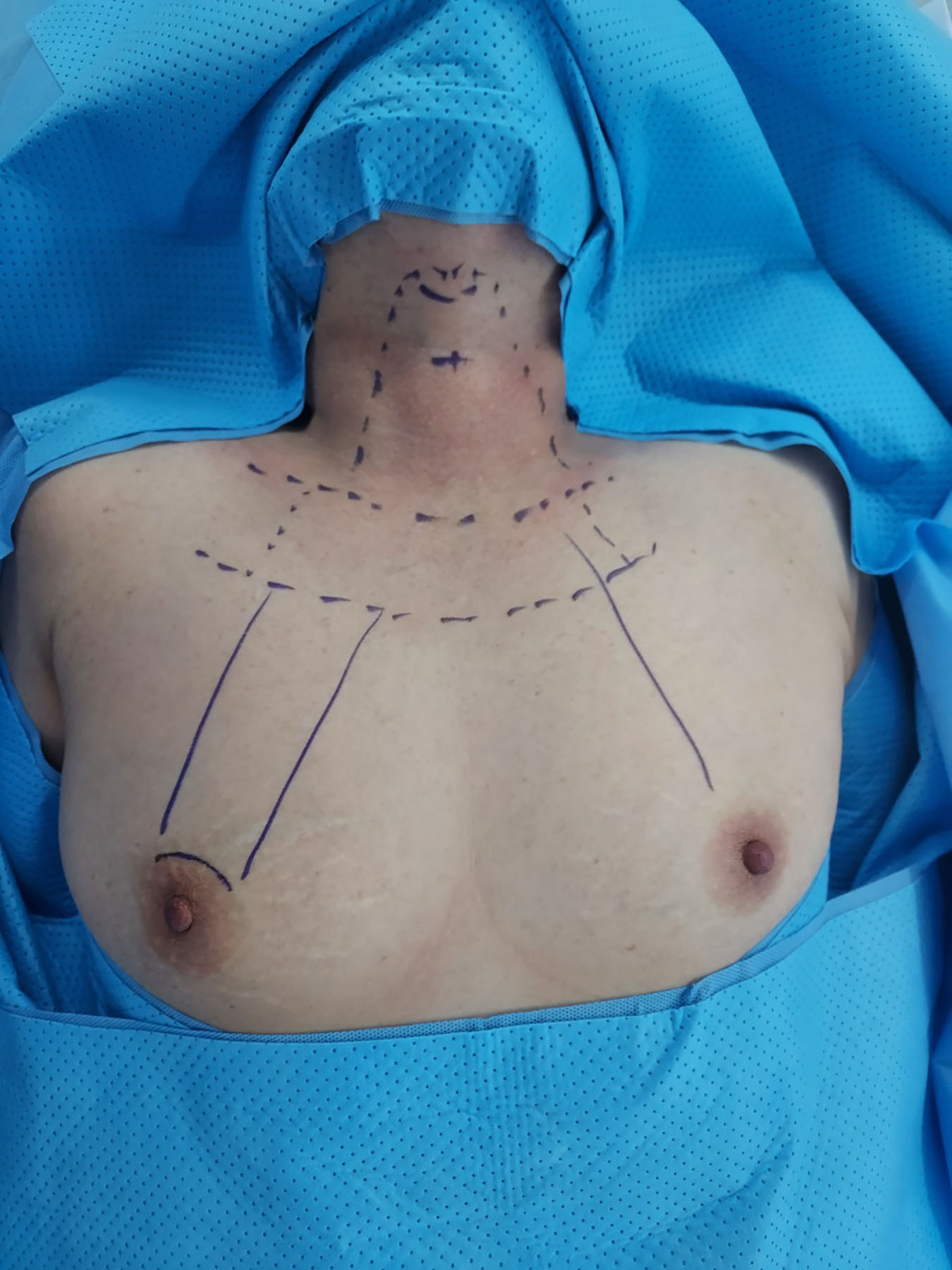
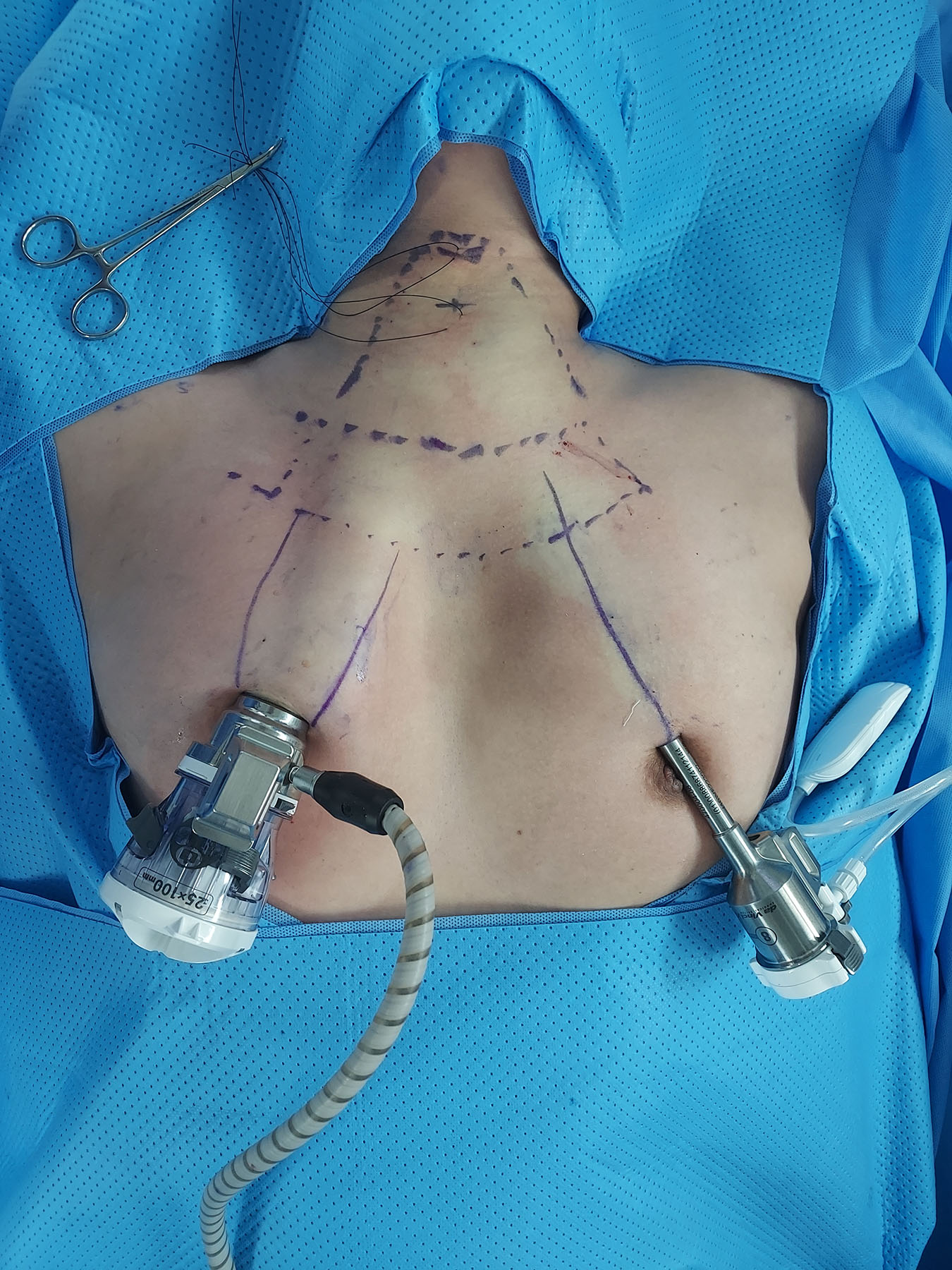
In this procedure, patients are positioned with their necks extended under general anesthesia. Intraoperative neuromonitoring is conducted using a NIM-3.0 endotracheal tube (Medtronic). The blueprint for the flap can be seen in Fig. 1. The da Vinci SP robot is docked through a 3-cm incision in the right areola, which allows access to both thyroid glands. An additional small incision in the left areola served as an auxiliary port. This port accommodates an 8-mm metal trocar, similar to those used in the da Vinci Xi, as illustrated in Fig. 2. A subcutaneous flap is created, and a suction irrigation tube or gauze ball is inserted through this port.
The process of creating a subcutaneous flap is divided into two steps. First, a 1:200,000 epinephrine saline solution is injected into the subcutaneous area, a 3-cm incision is made on the right areola, and blunt dissection is performed using a Mosquito-Kelly-vascular tunneler. This procedure mirrors the existing BABA technique, except that the axillary area is not accessed. Next, a 15-mm trocar is inserted into the right areola and the da Vinci SP camera is introduced through it. Further flap dissection is completed using an advanced bipolar device through the left areolar port. The flap is dissected beyond the head of the clavicle, along the sternocleidomastoid muscle and strap muscles, up to the upper level of the thyroid cartilage.
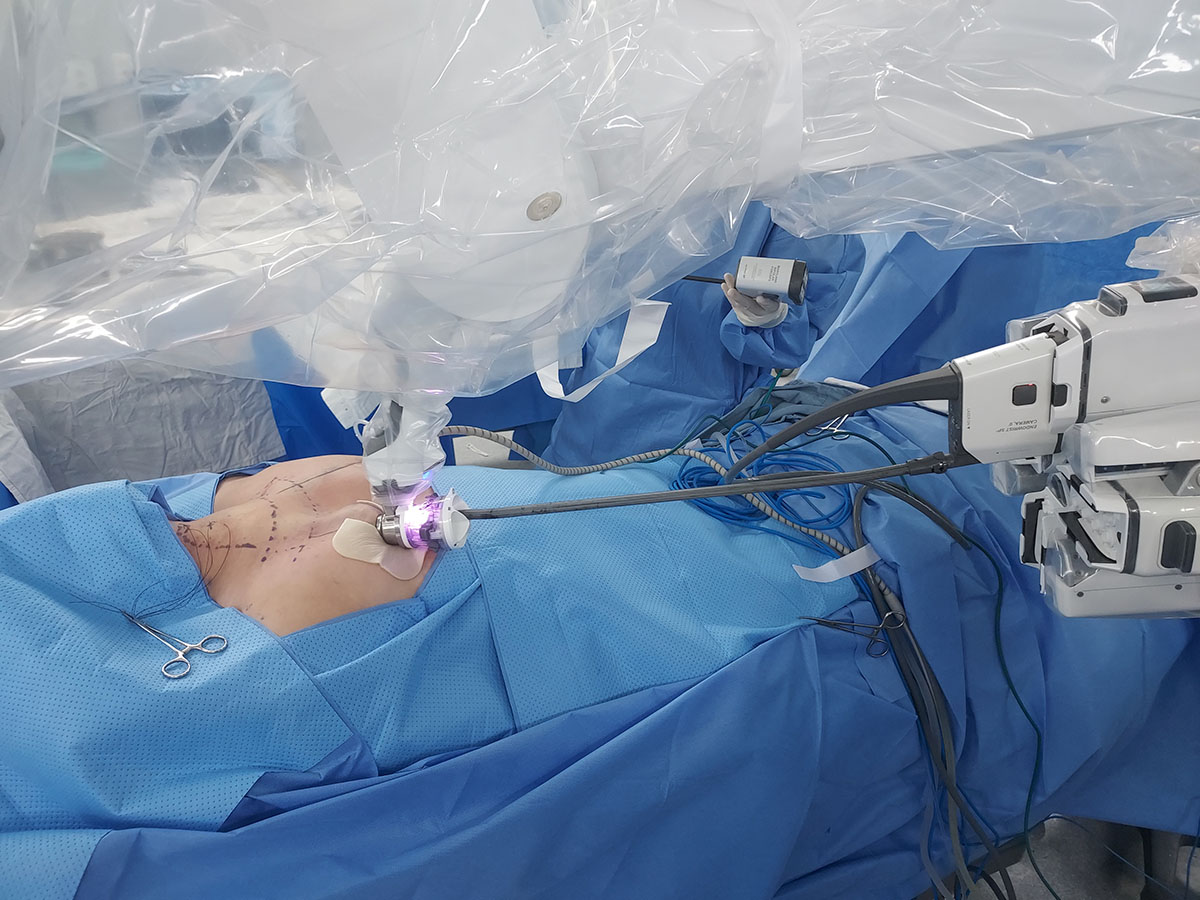
After the flap is completed, the robot is docked through the right breast incision, as shown in Fig. 3. The procedure utilizes two Maryland bipolar forceps (MBF) and one Cadiere forceps (CF). By setting the camera to Cobra mode, a 30° view identical to that of the conventional da-Vinci Xi 30° camera is achieved. The MBF is employed with both hands to split the midline of the strap muscles, revealing the trachea and thyroid gland. The thyroid isthmus is severed using the MBF. Then, the CF is used to grasp the isthmus and pull the thyroid in the opposite direction, detaching the strap muscle from the thyroid gland. The thyroid gland is elevated toward the medial-superior side with the CF and the recurrent laryngeal nerve is located in the level 6 area. When searching for the nerve, the nerve monitor cable is attached to the MBF. It is crucial to preserve the nearby lower parathyroid gland while safeguarding the nerve. The thyroid gland is dissected from the trachea side, ensuring the preservation of both the nerve and the parathyroid gland. Caution should be exercised when dissecting the ligament of Berry, as it is the thickest. The cricothyroid space is opened, and the superior thyroid vessels are coagulated. During the removal of the superior thyroid area, preservation of the upper parathyroid gland is essential. This completes the thyroid lobectomy. The central lymph node may be dissected while locating the recurrent laryngeal nerve or after the thyroidectomy. The surgical specimen is placed in a plastic bag and removed through the da Vinci SP port. The contralateral thyroidectomy and lymph node dissection are performed similarly, after re-docking the da Vinci SP through the same port. Post-surgery, a closed drain is inserted through the right areolar incision, and the procedure is concluded with a skin bond after a Vicryl intradermal suture. The complete procedure for the SPRA total thyroidectomy with central lymph node dissection is demonstrated in the provided video clip.
This study received ethical approval from the Institutional Review Board (IRB) of the Inha University Hospital (IRB number: 2024-05-014).
Discussion
The SPRA thyroid surgery is an evolution of the BABA method, offering a more minimally invasive approach by eliminating the need for subcutaneous flap dissection in the bilateral axillary area. The SPRA method facilitates easy access to both thyroid glands through a top-down vision and does not require changing the robot docking based on the lesion side [5]. Additionally, the procedure allows upward movement from the central lymph node area, enabling the monitoring of the bilateral recurrent laryngeal nerve with a neuromonitoring system connected to the robot’s bipolar grasper. The parathyroid glands are also more easily identified, benefiting from the fine instruments and the Firefly fluorescence function [6]. The surgical instruments of the da Vinci SP are significantly smaller (5 mm in diameter compared to 8 mm), allowing more precise dissection than those used in the da Vinci Xi. However, a notable limitation of the da Vinci SP system at present is the lack of advanced energy equipment, such as harmonic scalpels or vessel sealers.
We anticipate that SPRA thyroid surgery will gain popularity as more surgical experience is accumulated and as further comparative studies are conducted with BABA or open surgery, supporting its surgical and oncologic outcomes.
Notes
Disclosure
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: JWY; Data curation: SML, HOH; Writing–original draft: MHS, SML; Writing–review & editing: JWY.