Laparoscopic Right Hemicolectomy with an Inferior Approach: How I Do It
Article information
Abstract
Since its introduction in the early 1990s, laparoscopic colorectal surgery has been extensively validated through randomized controlled trials, establishing its safety and efficacy from oncological and technical standpoints. Laparoscopic right hemicolectomy (LRHC) procedures exhibit variability in dissection extent and initiation sites. Complete mesocolic excision is essential in LRHC, involving precise dissection along embryologic planes and varying in lymph node dissection extent (D2 or D3). Other variations in LRHC include the use of the medial approach (or superior mesenteric vein [SMV]-first approach), where dissection starts along the SMV, the lateral approach (or inferior approach), starting with meso-ileal and retroperitoneal dissection, and the superior approach, initiated by separation of the omentum and transverse colon. This paper presents a case of LRHC for ascending colon cancer using an inferior approach. The procedure included trocar placement, followed by inferior, superior, and medial dissection phases, concluding with specimen extraction and extracorporeal anastomosis. With a standardized procedure, mastery of diverse approaches (inferior, medial, and superior) remains crucial, as the most appropriate method varies among cases.
Introduction
Since its inception in the early 1990s, laparoscopic colorectal surgery has gained widespread acceptance due to its proven oncological and technical safety [1-3]. Favorable outcomes have led to a significant increase in the use of laparoscopic approaches for colorectal cancer (CRC), with rates rising from 50% in 2008 to nearly 80% in 2018 [4,5]. In performing laparoscopic right hemicolectomies (LRHCs), complete mesocolic excision (CME) is considered vital. CME requires fine dissection following the embryologic tissue planes which results in excision of the entire tumor-associated mesocolon [4]. CME procedures vary in the extent of lymph node dissection, classified as D1, D2, or D3. D2 dissection does not require the exposure of superior mesenteric vein (SMV), whereas D3 lymph node dissection necessitates the full dissection of lymph nodes along the SMV [5]. Applying D3 dissection or CME for all clinical stages of CRC is an ongoing discussion [6,7].
There are other variations on performing LRHC based on the dissection initiation point. The medial approach (or SMV-first approach) starts by dissection of mesocolic along the SMV, while the lateral approach (or inferior approach) begins with meso-ileum and retroperitoneum dissection. Additionally, the superior approach starts with the separation of the omentum and transverse colon. This technical paper aims to demonstrate a standardized method of LRHC established in a large-volume nationwide cancer center.
Case Presentation
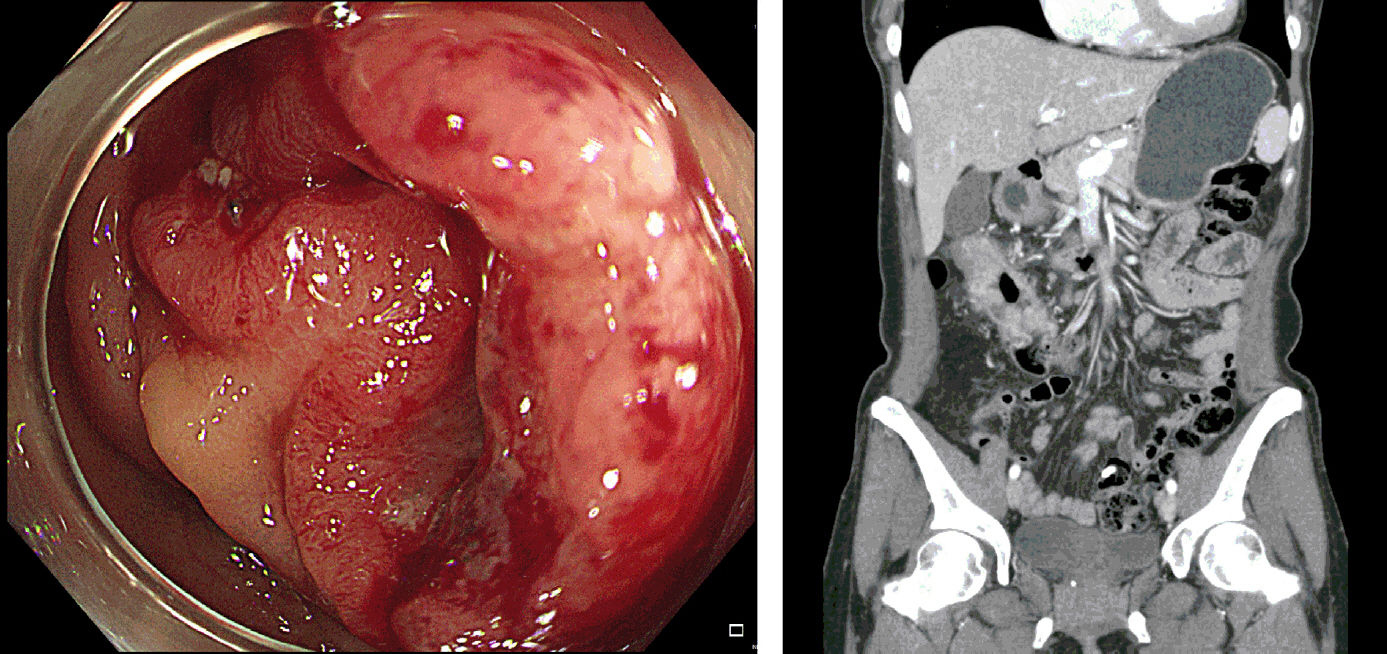
A 44-year-old female was diagnosed with advanced proximal transverse colon cancer during screening colonoscopy. Computed tomography scans revealed a clinical T3 to T4a with node-positive tumor without signs of distal metastasis (Fig. 1). The institutional review board approval was waived.
Surgical procedures
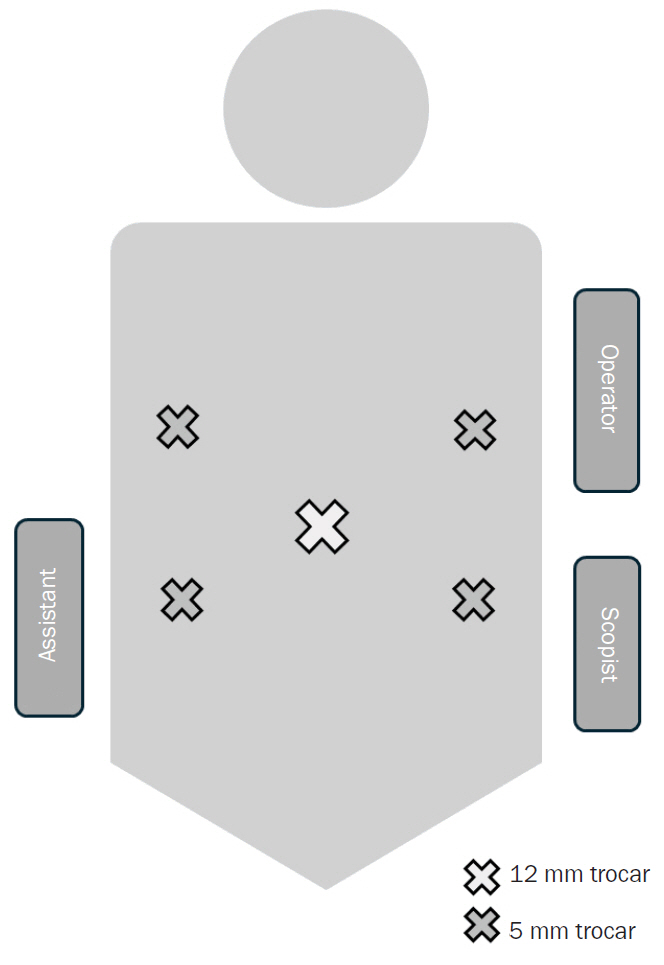
The patient was positioned supine. Five trocars, including one 12-mm camera port and four 5-mm ports, were initially placed (Fig. 2). The umbilical trocar was later extended for specimen extraction and extracorporeal anastomosis. After trocar insertion, the position was adjusted to a left-tilted Trendelenburg position, with the surgeon and scopist on the left and the assistant on the right.
Dissection began inferiorly with cecum mobilization, progressing superiorly towards the hepatic flexure along Toldt’s fascia. It is important to dissect along the proper layer as a deeper level of dissection may result in ureter or gonadal vessels injury. The medial aspect of Toldt’s dissection plane will reveal the duodenum with mesocolon being separated from the duodenum meticulously, and further dissection reveals the pancreas head. Full mobilization of the ascending colon up to the inferior aspect of the hepatic flexure and medial dissection up to the duodenum or pancreas head are landmarks where the inferior dissection phase is completed.
Subsequently, the patient is repositioned to a slightly reverse Trendelenburg position for the superior phase. Omental division initiates usually at the mid-transverse colon where division reveals the lesser sac. The omentum was divided and dissection continued towards the hepatic flexure. Careful attention is needed when dissecting near the right gastroepiploic vessels as the layer between the mesocolon, omental bursa, and pancreas may be fused and difficult to distinguish, causing injury in nearby structures such as the pancreas, gastroepiploic or infra-pyloric vessels, gastroduodenal vein or trunk of Henle, or duodenum. Once these structures are identified and safely isolated, further dissection towards the hepatic flexure along the fascial layer is done with relative ease. Further medial dissection may reveal the accessory right colic vein, with anatomic variations. Separation of the duodenum and pancreas head marks completion of the superior dissection phase.
The medial phase starts with identification of the ileocolic pedicle. While typically a groove forms from the mid-mesocolon towards the cecum, a useful landmark is to find the thin layer of mesocolon (“the anatomic sail”) which covers the duodenum area. The ileocolic pedicle lies inferiorly to this structure with its vessels resembling the mast of the sail. The dissection starts by exposing the left border of the SMV inferiorly to the ileocolic vessels continuing the dissection superiorly to reveal the ileocolic root. The vessels are ligated and cut, progressing superiorly to expose the SMV. Further exposure reveals the right colic vessels, also ligated. Dissection between the transverse colon and inferior border of the pancreas exposes the lesser sac with the remaining pedicle containing the middle colic vessels. This concludes the medial dissection, with the specimen externalized via the mini-laparotomy wound for extracorporeal anastomosis. All procedures are succinctly demonstrated in Video 1.
Postoperative courses
The total operative time was 107 minutes. The estimated blood loss was less than 50 mL without any transfusion perioperatively. Postoperative recovery was uneventful, with the patient discharged on the 7th postoperative day. The final pathology report revealed a pT3N0Mx stage, with a maximum tumor size of 5.2 cm and 44 lymph nodes yielded. The patient was followed with routine surveillance without adjuvant chemotherapy.
Discussion
This paper outlines an LRHC with lymphadenectomy along the SMV for ascending colon cancer with extracorporeal anastomosis, the preferred surgical treatment at our institute for ascending and proximal transverse colon cancer. Generally, in our institute, D2 plus principal lymph node dissection is considered for right-sided colon cancer. Although this study demonstrated the inferior approach technique, our surgeons are proficient with the inferior, medial, and superior approaches. It is important that colorectal surgeons be able to proficiently perform LRHC with diverse approaches, as the most appropriate method can vary by patient case. The inferior approach is convenient in that the surgeon has clear vision of duodenum and the mesocolon can be safely lifted without injuring the duodenum. However, in patients with obstructive lesions and dilated small bowel, starting from the inferior approach is difficult due to limited space which hinders the upheaving of meso-ileum. For these patients, medial approach is more suitable as it can be performed with relatively less working space. Conversely, when the tumor is in the proximal ascending colon and partly adhesive with the duodenum, both the medial and inferior approach may be difficult to initiate, prompting the surgeon to start from a superior approach. One should bear in mind, there is no stereotypical surgery and dexterous laparoscopic skills combined with in-depth understanding of anatomical planes allow surgeons to perform tailor-made operations for each patient’s unique situation.
Notes
Disclosure
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: YIK, HL; Data curation: YIK, MHK; Investigation and Formal analysis: YIK, HL; Methodology and Software: HL, MHK; Writing–original draft: YIK, HL; Writing–review & editing: MHK.