Abstract
- Diaphragmatic hernias, whether congenital or acquired, often require surgical intervention to prevent life-threatening complications. The use of biologic mesh has gained increasing attention due to its favorable integration with host tissue and lower recurrence rates. This article presents a reproducible and effective method for diaphragmatic hernia repair using a biologic mesh via a laparoscopic approach. The technique emphasizes anatomical restoration and durable fixation, while minimizing tension and postoperative complications.
-
Keywords: Diaphragmatic hernia, Surgical mesh, Hernia, diaphragmatic, Herniorrhaphy, Hernia, hiatal
Introduction
Diaphragmatic hernias result from a defect or disruption in the diaphragm, allowing abdominal contents such as the stomach and colon to migrate into the thoracic cavity. Traditional repairs usually involve primary suturing, but large or tension-prone defects often require additional reinforcements such as mesh, which can be synthetic or biologic in nature. Biologic mesh (hereafter, simply biomesh), which is an allograft or xenograft mesh biologically compatible with surrounding tissues, has enabled more robust repairs, particularly in contaminated fields or in cases where synthetic meshes are contraindicated [1,2]. In comparison to primary closure, biomesh repair is known to have a lower recurrence rate of paraesophageal hernias (9% vs. 24%) [3]. Through this paper and video, I would like to share our experience of using biomesh for effectively repairing a large delayed-presentation diaphragmatic hernia.
Case Presentation
An 83-year-old female with abdominal pain and vomiting was referred to the gastrointestinal surgery department due to a large diaphragmatic hernia causing gastric outlet obstruction. The patient’s body mass index was 21.1 kg/m2 with a height of 143.0 cm and weight of 42.4 kg. Her medical history included diabetes mellitus and hypertension with no history of surgery or trauma, family disease, alcohol consumption, and smoking.
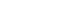
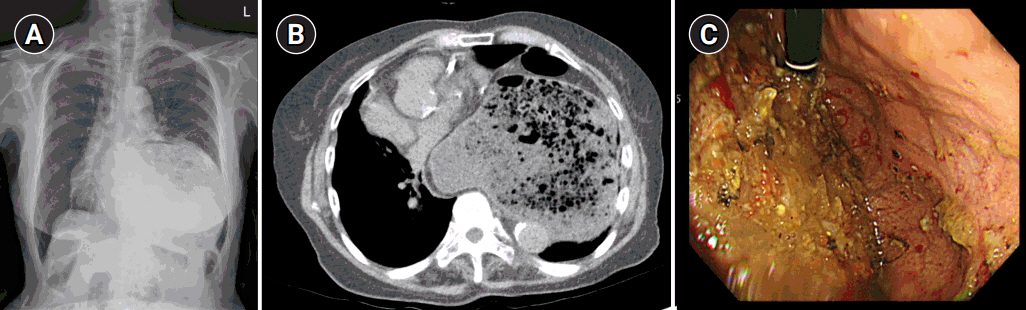
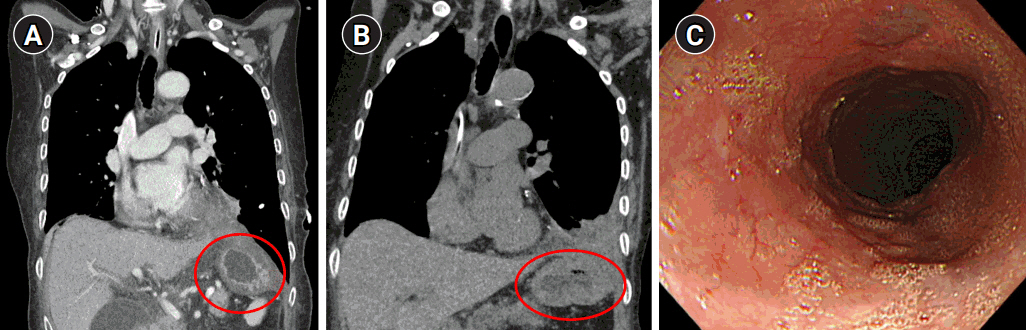
Preoperative chest radiography and computed tomography (CT) confirmed a very large left-sided diaphragmatic hernia, approximately 5.5 cm in diameter, of the stomach and transverse colon (Fig. 1A, B). Esophagogastroduodenoscopy (EGD) showed an abnormal stomach body shape with large amount of food contents, making further evaluation difficult (Fig. 1C). After discussing the results and risk with the patient and her family, the patient agreed to proceed with surgical reduction.
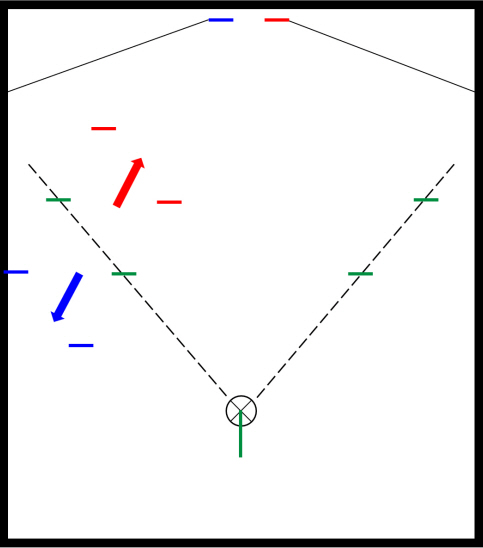
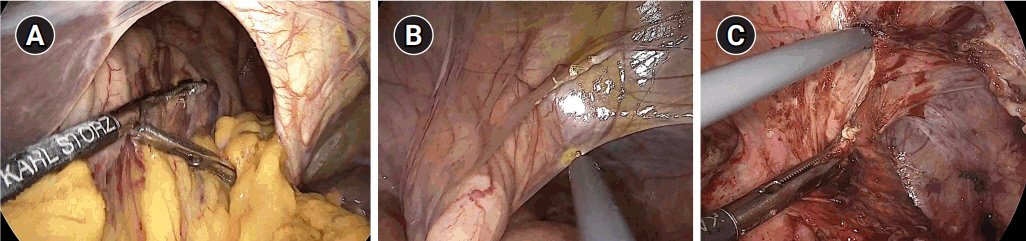
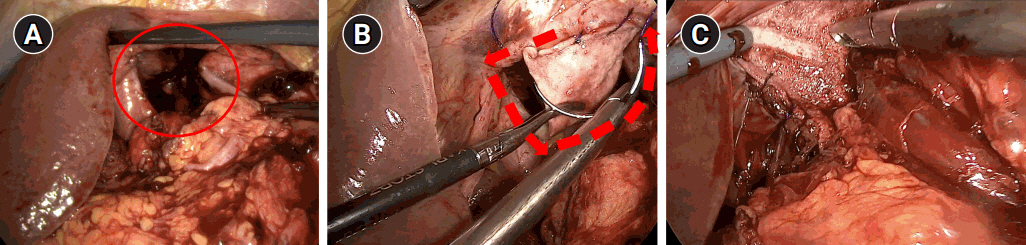
After general anesthesia, laparoscopic exploration (as shown in the video) confirmed that the stomach and transverse colon had been herniated into the thoracic cavity. The ports were positioned similar to those of laparoscopic gastrectomy, but with the operator’s ports placed slightly more medial and upwards (Fig. 2) considering the left-sidedness of the diaphragmatic hernia. Had the diaphragmatic hernia been right-sided, the author would have placed the ports slightly more lateral and lower. Using laparoscopic graspers, the stomach and transverse colon were carefully pulled back into the abdominal cavity (Fig. 3A). Adhesions around the stomach, which were severe most likely due to the chronic herniation, were carefully dissected using a laparoscopic electrode device (Fig. 3B). After meticulous dissection, the stomach was finally mobilized from the thorax and successfully pulled back into the abdominal cavity. Hernia sac was then meticulously dissected, allowing the esophagus to be identified (Fig. 3C). During this procedure, the distal esophagus was injured, creating an iatrogenic hole, which was repaired using 3-0 continuous absorbable barbed suture (Monofix®; Hanmi Healthcare) (Fig. 4). Endoscopic stent or endo-vac therapy, which have been reported to be effective in treating esophageal perforations after paraesophageal hernia operations, was planned to be used postoperatively if necessary [4,5].
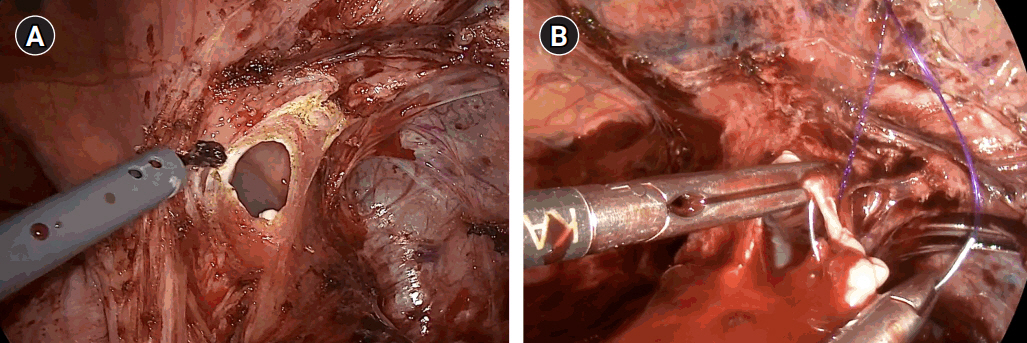
The diaphragm defect was measured to be approximately 5 cm in diameter, which was to be repaired using a biomesh (Fig. 5A). The biomesh chosen for this case was a human acellular dermal matrix measuring 3.0×4.0 cm in dimension with a thickness of 1.0–2.0 mm (SC Derm®; DOF Inc.). Using this biomesh, the diaphragm was augmented and repaired by circumferentially anchoring the biomesh to the diaphragm wall using 3-0 continuous Monofix® (Fig. 5B). After checking that there was no significant tension, no residual herniation or bleeding and that the lung expanded back well, the operation was deemed successfully complete and the patient was extubated uneventfully (Fig. 5C).
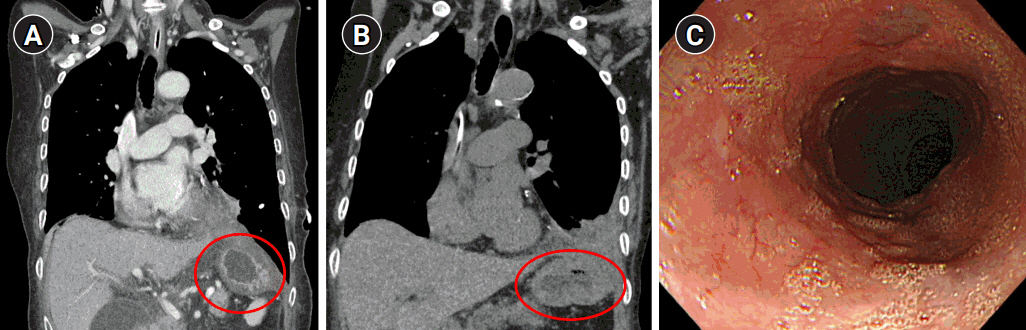
Following surgery, the patient underwent endo-vac therapy and endoscopic stent insertion due to leakage from the esophageal injury site. After approximately three months of nutritional support and conservative care, follow-up CT and EGD showed that there was no herniation and that the perforation site had been sealed with no signs of fistula (Fig. 6).
This case report was exempted from review by the Institutional Review Board of Asan Medical Center. Informed consent from the patient was waived due to anonymized data.
Discussion
In this rare and large diaphragmatic hernia case, laparoscopic repair was successfully performed using biomesh augmentation. Laparoscopic approach in comparison to open surgery not only offers enhanced visualization of the surgical field, which is crucial for safe and successful repair, but it is also known to significantly reduce hospital stay and recurrence rate [6,7]. Since the hernia defect measured over 5 cm, we decided to use a biomesh made from human acellular dermal matrix with dimension of 3.0×4.0 cm, which was sufficient for tension-free anchorage to the diaphragm wall. We chose biomesh over synthetic mesh because of the potential risk of mesh migration and erosion into the surrounding esophagus and lungs, which could be life threatening [7].
The surgeon must be aware of the following factors prior to entering the operating room. First, severe adhesions exist around the herniated contents due to prolonged herniation, as a result of asymptomatic and delayed presentation [7,8]. This creates a problem because adhesiolysis in this space can easily lead to iatrogenic injury of the esophagus. Since, esophageal injury is quite common in this surgical field, the surgeon must be ready to repair an unexpected esophageal injury using the proper suture materials and technique [5]. Before ending the operation, the surgeon must confirm that there is no residual herniation or bleeding, and that the lung expansion is recovered. Second, considering the circular shape of diaphragmatic hernias, biomesh must be meticulously sutured 360° around the defect. Keeping in mind that this is a laparoscopic approach, the surgeon must be well prepared and confident in laparoscopic suture.
In conclusion, biomesh repair can be effectively and safely applied for large diaphragmatic hernias which cannot be simply repaired by primary sutures. In this context, biomesh provides a scaffold that facilitates tissue integration while lowering recurrence and avoiding complications associated with synthetic materials [1]. In contaminated or chronic cases, such as post-traumatic diaphragmatic hernias, biomesh demonstrates lower infection rates and improved healing by reducing the risk of erosion into the surrounding organs [2]. While long-term data is still evolving, biomesh appears promising for not only complex hernia repairs, such as patients with prior infections, comorbidities, or trauma history, but also for elective surgeries dealing with large hernias.
Disclosure
In-Seob Lee (editor-in-chief) and Sa-Hong Min (associate editor) were not involved in the evaluation or decision-making process of this article, which adhered to decisions made by independent reviewers. There are no other potential conflicts of interest relevant to this article.
Acknowledgments
We would like to thank the operating room staff of Asan Medical Center for their assistance during the procedure.
Author contributions
Conceptualization: JY, CSG; Data curation: JY, CSG; Formal analysis: JY, CSG; Investigation: JY, CSG; Methodology: JY, CSG; Supervision: CSG, CSK, SHM, ISL, MWY, JHY, BSK; Writing–original draft: JY, CSG, BOS, CSK, SHM, ISL, MWY, JHY, BSK; Writing–review & editing: CSG, CSK, SHM, ISL, MWY, JHY, BSK.
Fig. 1.Preoperative imaging evaluation. (A) Chest radiography showing a left-sided diaphragmatic hernia, (B) abdominal computed tomography showing a diaphragmatic hernia measuring approximately 5.5 cm in diameter. (C) Esophagogastroduodenoscopy showing a large amount of food contents due to gastric outlet obstruction.
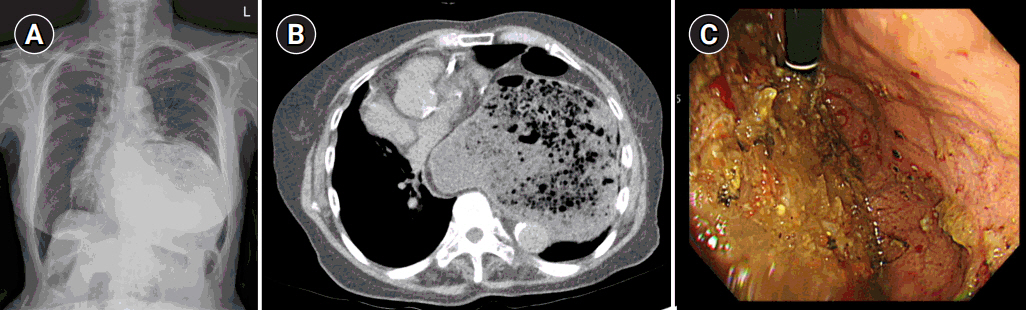
Fig. 2.Laparoscopic port positions. The typical gastrectomy ports are shown in green, while red shows the author’s preferred positions for left-sided diaphragmatic hernia and blue represents the positions for right-sided diaphragmatic hernia.
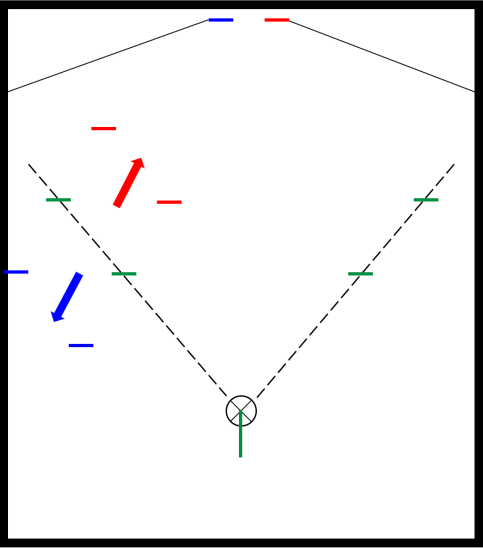
Fig. 3.Laparoscopic exploration and reduction. (A) Reduction of the herniated stomach and transverse colon, (B) adhesiolysis around the stomach, and (C) circumferential dissection of the hernia sac.
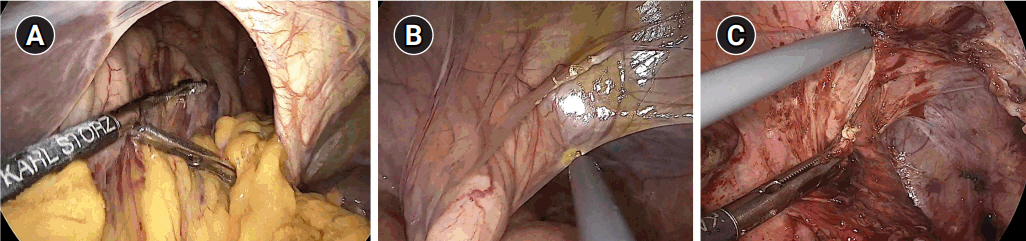
Fig. 4.Esophageal injury. (A) Iatrogenic injury caused during dissection of the hernia sac. (B) Continuous suture repair using 3-0 Monofix®.
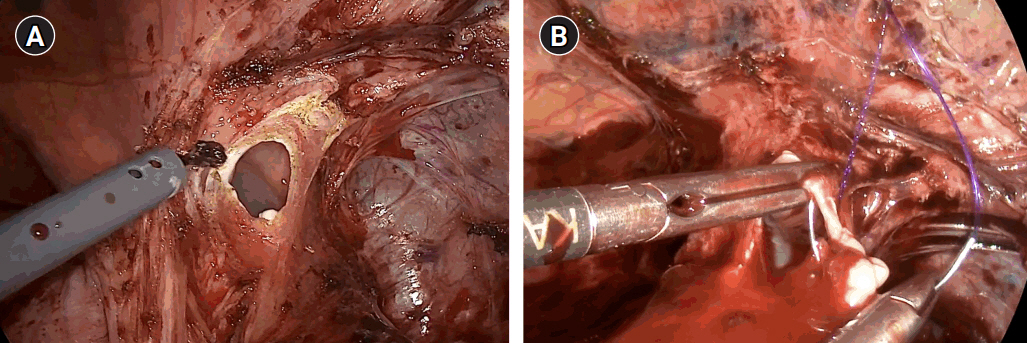
Fig. 5.Biomesh repair procedure. (A) Evaluation of the herniated space after reduction (circle). (B) 360° continuous suture fixation of the biomesh to the diaphragm (dotted arrow line). (C) Confirmation of repair.
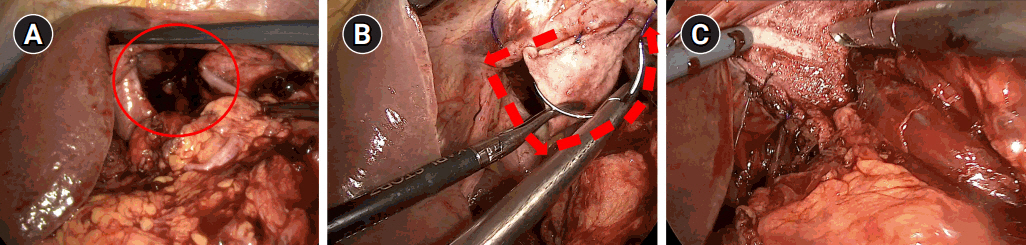
Fig. 6.Follow-up computed tomography at postoperative day 17 (A) and 3 months (B), showing no herniation of the stomach and bowel contents (circles). (C) Esophagogastroduodenoscopy finding at postoperative 3 months, showing no clear trace of the previous perforation site.
REFERENCES
- 1. Antoniou SA, Pointner R, Granderath FA, Köckerling F. The use of biological meshes in diaphragmatic defects: an evidence-based review of the literature. Front Surg. 2015;2:56.ArticlePubMedPMC
- 2. Giuffrida M, Perrone G, Abu-Zidan F, Agnoletti V, Ansaloni L, Baiocchi GL, et al. Management of complicated diaphragmatic hernia in the acute setting: a WSES position paper. World J Emerg Surg. 2023;18:43.ArticlePubMedPMCPDF
- 3. Oelschlager BK, Pellegrini CA, Hunter J, Soper N, Brunt M, Sheppard B, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg. 2006;244:481-490.ArticlePubMedPMC
- 4. Ko E, O-Yurvati AH. Iatrogenic esophageal injuries: evidence-based management for diagnosis and timing of contrast studies after repair. Int Surg. 2012;97:1-5.ArticlePubMedPMCPDF
- 5. Botha AJ, Di Maggio F. Management of complications after paraesophageal hernia repair. Ann Laparosc Endosc Surg. 2021;6:38.Article
- 6. Hietaniemi H, Järvinen T, Ilonen I, Räsänen J. Congenital diaphragmatic hernia in adults: a decade of experience from a single tertiary center. Scand J Gastroenterol. 2022;57:1291-1295.ArticlePubMed
- 7. Jones EK, Andrade R, Bhargava A, Diaz-Gutierrez I, Rao M. Surgical management of delayed-presentation diaphragm hernia: a single-institution experience. JTCVS Tech. 2022;13:263-269.ArticlePubMedPMC
- 8. Yin V, Pagteilan J, Atay SM, David EA, Kim AW, Wightman SC. Esophagectomy for permanent mesh migration after transthoracic hiatal hernia repair. Ann Thorac Surg. 2022;114:e89-e91.ArticlePubMed
Citations
Citations to this article as recorded by

 , Chung Sik Gong1
, Chung Sik Gong1 , Ba Ool Seong2
, Ba Ool Seong2 , Chang Seok Ko1
, Chang Seok Ko1 , Sa-Hong Min1
, Sa-Hong Min1 , In-Seob Lee1
, In-Seob Lee1 , Moon-Won Yoo1
, Moon-Won Yoo1 , Jeong Hwan Yook1
, Jeong Hwan Yook1 , Beom Su Kim1
, Beom Su Kim1







 PubReader
PubReader ePub Link
ePub Link Cite this Article
Cite this Article
 KSSSG
KSSSG